Cardiac
Researchers are using human cardiac microtissues as a platform for predicting arrhythmia generation and cardiotoxicity in response to environmental chemicals and pharmaceutical drugs as an alternative to animal testing.
Cardiac
Researchers are using human cardiac microtissues as a platform for predicting arrhythmia generation and cardiotoxicity in response to environmental chemicals and pharmaceutical drugs as an alternative to animal testing.
Cardiac 3D Microtissues
- The Coulombe and Choi labs, in consultation with Dr. Mende, have developed 3D human heart microtissues for assessing cardiotoxicity risk of pharmaceutical drugs, industrial chemicals, and environmental toxicants.
- The platform technology uses cardiomyocytes derived from human-induced pluripotent stem cells (iPSCs) and human cardiac fibroblasts to enable tissue-like cellular interactions that increase predictive value of the model.
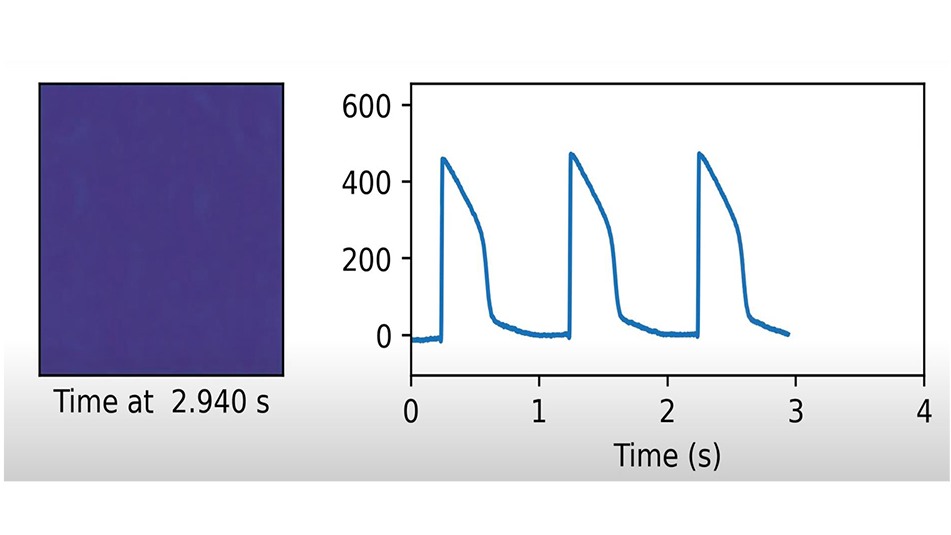
- Cardiotoxicity manifests as electrical disturbances (arrhythmias), as well as structural, calcium handling, contractile, and metabolic deficiencies.
- Use of sophisticated high-resolution, high-throughput optimal mapping techniques enable functional measurements of the voltage of the action potential and calcium of the intracellular calcium transient, identifying safe exposure levels and those that elicit arrhythmias.
- Human iPSC-derived cardiac microtissues show dose-dependent arrhythmia responses to high-risk drugs (like cisapride, which is no longer on the market), low-risk pharmaceutical compounds (like the cardiac drug ranolazine), and environmental toxicants (like bisphenol-A).
- This platform is an alternative to animal toxicity testing and may be a more predictive indicator of human arrhythmic cardiotoxicity when used in conjunction with computational in vitro to in vivo extrapolation approaches currently ongoing in collaboration with ScitoVation.
- Commercialization of the human cardiac 3D microtissues aims to fill a need in toxicity testing and risk assessment to be more sensitive and predictive of the responses of the human heart in order to preserve and protect cardiac safety.
 Play
Play
The electrical activity of multiple human stem cell-derived heart microtissues can be seen and measured by their flashes of fluorescent light. The rhythm of the heart microtissues is determined by tracing these flashes and is being used to identify toxic chemicals and drugs that alter this rhythm, thus creating arrhythmias.
Learn More
Kofron, C. M., Kim, T. Y., Munarin, F., Soepriatna, A. H., Kant R. J., Mende, U., Choi, B. R., Coulombe, K. L. (2021). A predictive in vitro risk assessment platform for pro-arrhythmic toxicity using human 3D cardiac microtissues. Scientific Reports,11(1), 10228. PMC8119415
Rupert, C.E, Kim T. Y., Choi B. R., Coulombe K. L. (2020). Human cardiac fibroblast number and activation state modulate electromechanical function of hiPSC-cardiomyocytes in engineered myocardium. Stem Cells International, 9363809. PMC7381987
Rupert, C. E., Irofuala, C., & Coulombe, K. (2020). Practical adoption of state-of-the-art hiPSC-cardiomyocyte differentiation techniques. PloS one, 15(3). PMC7064240
Desroches, B. R., Zhang, P., Choi, B. R., King, M. E., Maldonado, A. E., Li, W., Rago, A., Liu, G., Nath, N., Hartmann, K. M., Yang, B., Koren, G., Morgan, J. R., & Mende, U. (2012). Functional scaffold-free 3-D cardiac microtissues: a novel model for the investigation of heart cells. American Journal of Physiology Heart and Circulatory Physiology, 302(10), H2031–H2042. PMC3362102
Gerbin, K. A., Yang, X., Murry, C. E., & Coulombe, K. L. (2015). Enhanced electrical integration of engineered human myocardium via intramyocardial versus epicardial delivery in infarcted rat hearts. PLoS One, 10(7), e0131446. PMC4498815
Kofron, C.M., Mende, U. (2017). In vitro models of the cardiac microenvironment to study myocyte and non-myocyte crosstalk: bioinspired approaches beyond the polystyrene dish. Journal of Physiology, 595(12), 3891-3905. PMC5471366
Kofron, C. M., Kim, T. Y., King, M. E., Xie, A., Feng, F., Park, E., Qu, Z., Choi, B. R., & Mende, U. (2017). Gaq-activated fibroblasts induce cardiomyocyte action potential prolongation and automaticity in a 3D microtissue environment. American Journal of Physiolology Heart and Circulatory Physiolology, 313(4), H810-H827. PMC5668610
Kim, T. Y., Kofron, C. M., King, M. E., Markes, A. R., Okundaye, A. O., Qu, Z., Mende, U., & Choi, B. R. (2018). Directed fusion of cardiac spheroids into larger heterocellular microtissues enables investigation of cardiac action potential propagation via cardiac fibroblasts. PLoS One, 13(5), e0196714. PMC5929561
Investigators
-

Kareen Coulombe, PhD
Associate Professor of Engineering & Medical Science, Center for Biomedical Engineering, School of Engineering -

Ulrike Mende, MD, FAHA
Professor of Medicine, Department of Medicine -

Bum-Rak Choi, PhD
Associate Professor of Medicine, Department of Medicine