Tissue Model of Fibrosis
The Morgan lab has developed a new microtissue model that will uncover the underlying mechanism of connective tissue disorders.
Tissue Model of Fibrosis
The Morgan lab has developed a new microtissue model that will uncover the underlying mechanism of connective tissue disorders.
Ring-shaped 3D Microtissues: New Model of Fibrosis
- The Morgan lab has developed a new ring-shaped 3D microtissue model of fibrosis. Fibrosis is a disease of multiple organs including skin, liver, kidney, heart and lung where excess deposition of collagen by connective tissue cells results in scarring that severely compromises organ function.
- The ring-shaped microtissues are comprised of human fibroblasts, the cells of connective tissue that are responsible for the production of collagen as well as the other proteins that constitute the extracellular matrix (ECM).
- The ECM surrounds all cells and is largely responsible for the mechanical properties of tissues and organs. The pathological overproduction of collagen and ECM during fibrosis increases the stiffness of organs which adversely affects organ function.
- In the ring-shaped microtissue model, the human fibroblasts elongate, align and contract to form a 3D ring-shaped tissue with a histology that mimics human connective tissue. The fibroblasts synthesize abundant amounts of collagen and other ECM proteins and organize these proteins into complex fibers that encircle the ring.
- Using a mechanical testing instrument, we’ve measured the stiffness and tensile strength of the rings. We’ve shown that addition of TGF-b1, a growth factor implicated in promoting fibrosis, increases the stiffness and tensile strength of the rings. Conversely, we’ve shown that addition of the drug SB-431542 which inhibits TGF-b1 actions, decreases the stiffness and ultimate tensile strength of the rings.
- Because we can carefully measure changes to the stiffness of the ring-shaped microtissues, we now have a new model based on human cells that more closely mimics the increase in stiffness that adversely affects fibrotic organs.
- We are using ring-shaped microtissues to measure the effects of chemicals and growth factors that may promote fibrosis. And, we are using ring-shaped microtissues to measure the effects of drugs that may be useful for the treatment of fibrosis.
After human fibroblasts are seeded into a specially designed ring-shaped mold, the cells aggregate and self-assemble to form a 3D ring-shaped tissue in less than 24 hours. Stable for over 4 weeks, the ring-shaped tissues synthesize collagen and other proteins of the extracellular matrix (ECM).
Ring-shaped tissues of human fibroblasts synthesize abundant amounts of collagen and organize the collagen into fibers. The 3D organization of the fibers of rings are well developed by day 14 and are visualized using second harmonic generation (SHG microscopy).
A ring-shaped tissue is stretched by a mechanical testing instrument until it breaks at its ultimate tensile strength. As the tissue is stretched, the instrument simultaneously measures elongation of the ring (strain) as well as the force required to stretch the ring (stress). From this stress/strain curve, tissue stiffness is calculated. TGF-B1 increases stiffness and tensile strength, whereas the drug SB-431542 which inhibits TGF-B1 actions, decreases stiffness and tensile strength.
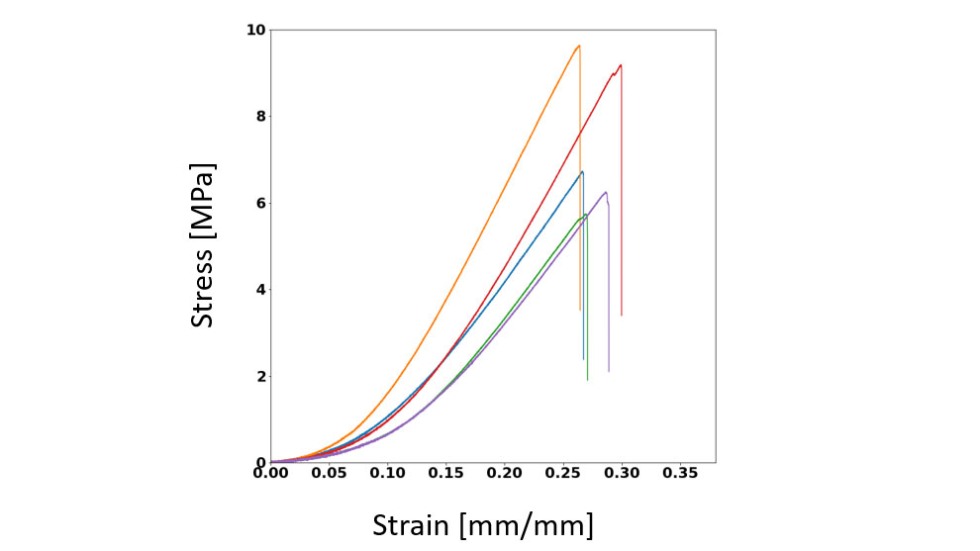
A ring-shaped tissue is stretched by a mechanical testing instrument until it breaks at its ultimate tensile strength. As the tissue is stretched, the instrument simultaneously measures elongation of the ring (strain) as well as the force required to stretch the ring (stress). From this stress/strain curve, tissue stiffness is calculated. TGF-B1 increases stiffness and tensile strength, whereas the drug SB-431542 which inhibits TGF-B1 actions, decreases stiffness and tensile strength.
Learn More
Wilks, B.T., Evans, E.B., Howes, A., Hopkins, C.M., Nakhla, M.N., Williams, G. & Morgan, J.R. (2022) Quantifying cell-derived changes in collagen synthesis, alignment, and mechanics in a 3D connective tissue model. Advanced Science. In press.
Investigators
-

Jeffrey Morgan, PhD
Donna Weiss ’89 and Jason Weiss Director of the Center for Alternatives to Animals in Testing, Professor of Medical Science, Department of Pathology and Laboratory Medicine